Notes and calculations spectrophotometric metal lon analysis experiment 35 prelaboratory assignment spectrophotometric metal ion analysis dale desk no lob sec for preparing the standard solutions in pant a 27 prepared ก เค pan a 1 how many millilan dual velme will be used pr 1 2 experimental procedure part a l.
Experiment 35report sheet spectrophotometric metal ion analysis.
Experimental procedure part a.
View lab report chem lab 35 from cp 2121 at the college at old westbury.
We begin with a description of the spectrophotometric experiment.
More practice with application s of beer s law 2.
Experiment 35 boratory assignment spectrophotometric metal ion analysis lab sec.
This is a group experiment.
Total volume will be used many milliliters 2.
Introduction this experiment involves the use of absorption spectrophotometry to quantify concentrations of three metal ions co ii cu ii and ni ii based on differences in the absorption spectra of edta complexes of the ions.
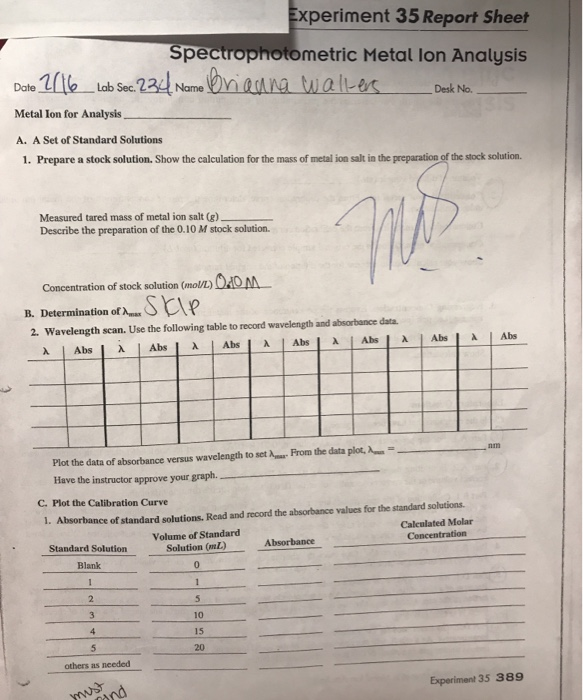
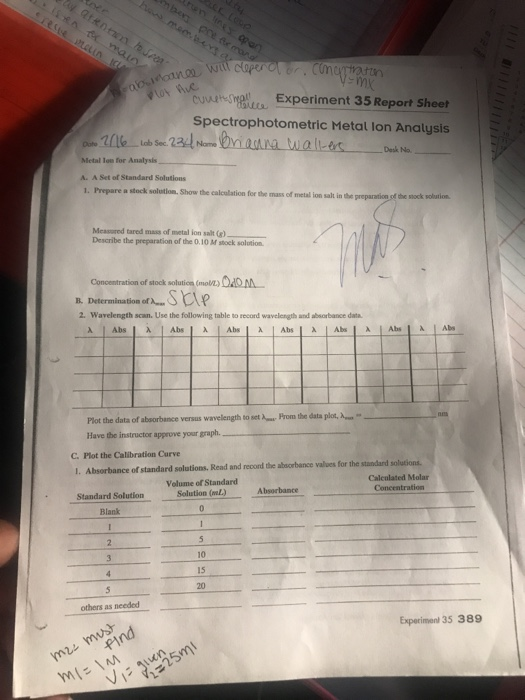
A set of standard solutions 1.
Solutions in part desk no.
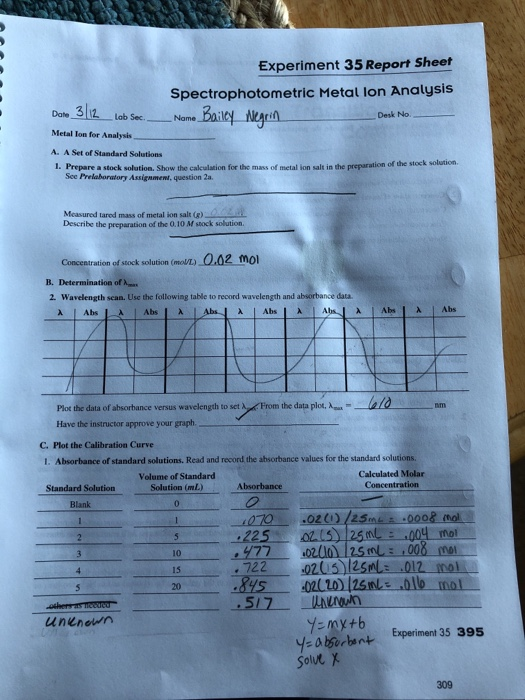
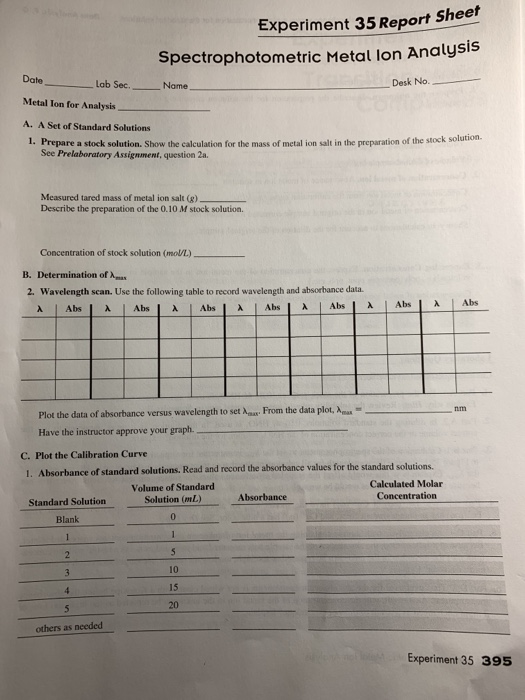
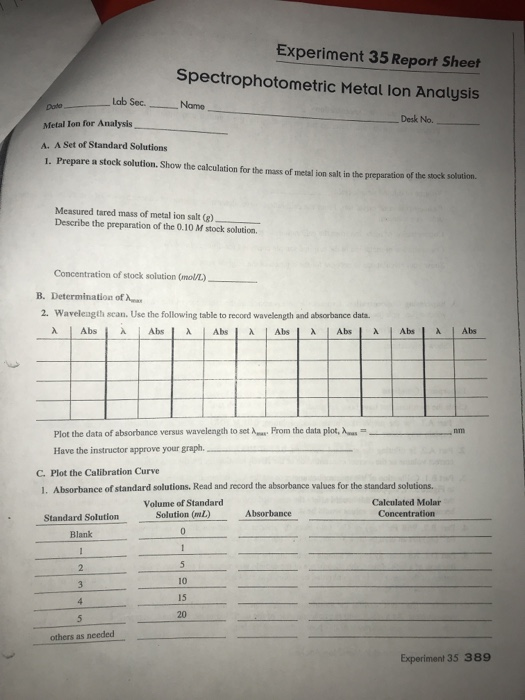
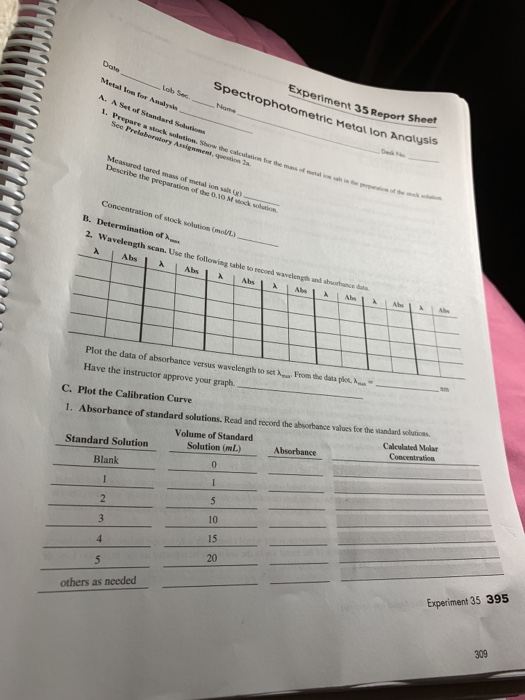
Experiment 35 report sheet spectrophotometric metal lon analysis date metal ion for analysis a.
Experiment 35 report sheet spectrophotometric metal ion analysis date 3 12 lab sec name metal ion for analysis a.
The purpose of performing this laboratory experiment was.
View lab report experiment 35 report sheet from chem 100 at silver creek high school.
Experiment 7 spectrophotometric iron analysis learning goals 1.
Experiment 35 report sheer spectrophotometric metal ion analysis j j date lab sec.
Spectrophotometric metal ion analysis introduction.
The inorganic analyte being considered in this particular analysis is phosphate and the interfering substance is arsenic.
Prepare a stock solution.
Prepare a stock solution.
2 0 theory the first portion of a spectrophotometric analysis consists of preparing six standard solutions each with a known phosphate.
Show the calculation for the mass of metal ion desk no.
Spectrophotometric analysis and to examine the effect of an interfering substance.
Show the calculation for the mass of metal ion salt in the preparation of the stock solution.
All groups in each laboratory will share one set of standard solutions.
Experiment the absorption of light of 522 nm wavelength by a sample solution will lead to an analysis for a trace amount of iron in an unknown sample.
Of the 100 ml of stock solution tha for preparing the l is be prepared for part ai.
Gain experience with pre treatment using a color developing agent introduction spectrophotometric methods of analysis are fast relatively simple and very widely applied.
Consider a sample of some solution contained in a small transparent vessel perhaps a test tube.
Salt in the preparation of the stock solution sce prelaboratory assigament question 2a.